With a team of more than 40 people, PHAGECON is a company dedicated to providing quality and excellency services to the pharmaceutical industry.
We present a wide range of services in regulatory affairs, pharmacovigilance, medical and scientific affairs and pharmaceutical affairs that enables us to follow the life cycle of companies of sector and their health products.
Our experience and operational flexibility allow us to offer our clients an overall and customized solution to any request related to regulatory, technical and scientific affairs:
- of any health product,
- of any activity of that sector,
- at any stage of development.
Our partners and clients have a competitive advantage over their competitors: they have the guarantee that all services hired will be carried out successfully, on time and will bring added value to their organizations. These companies are focused on their business aims, optimizing their resources while acquiring value-added services.
Become part of this committee group, contact us.
OUR TEAM
With more than 10 year of experience in the Pharmaceutical Industry, Catarina has started her career in INFARMED I.P. founding PHAGECON in 2006.
Since then, the company has seen its business grow constantly becoming one of the most prominent Pharmaceutical Consultancy Companies in Portugal
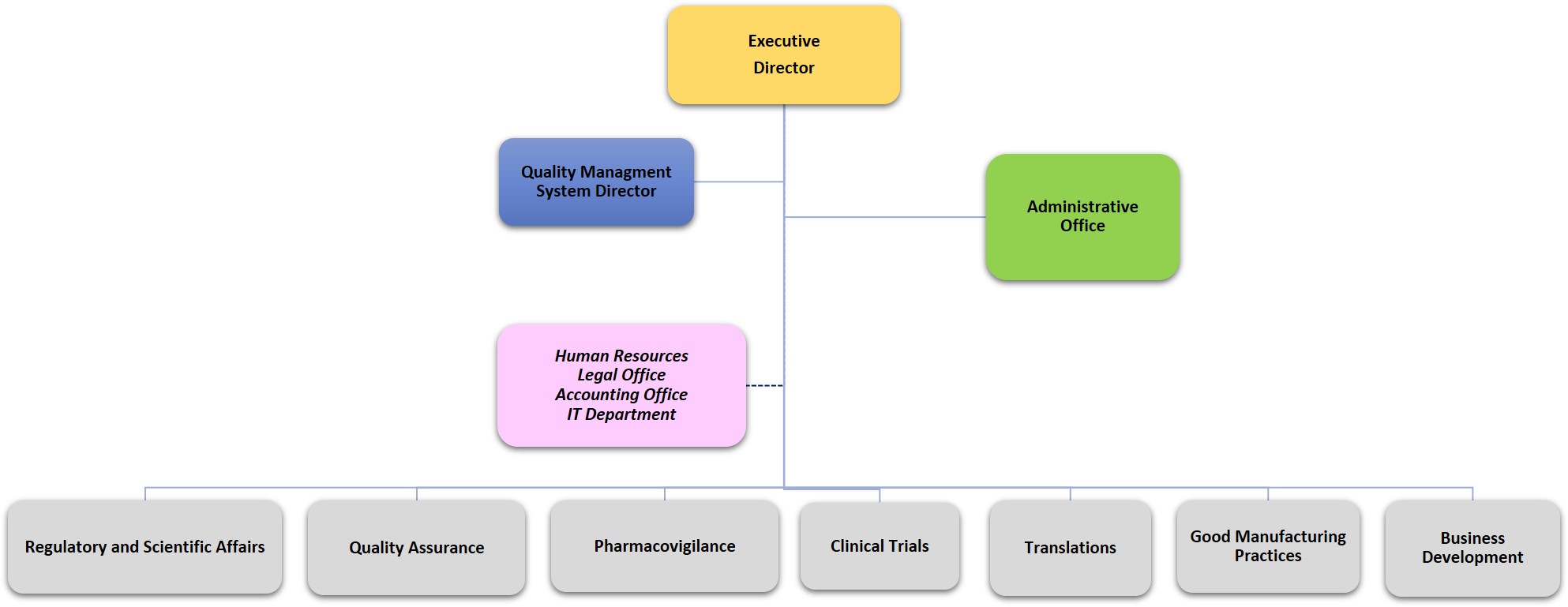
Organizational Chart
Performing business in 4 Continents













